
search
Jan 12, 2026
The pharmaceutical industry relies on dependable API manufacturing services to ensure that drug products reach patients safely and on time. Disruptions in the supply chain – whether from natural disasters, regulatory issues, or global crises – can delay production and impact patient care. Many companies address these risks by partnering with CDMOs that implement business continuity management (BCM) strategies to ensure supply from development through commercialization.


Business continuity management (BCM) refers to the proactive planning and processes that CDMOs use to maintain production and supply of active pharmaceutical ingredients (APIs) even during unexpected disruptions. In API drug development and manufacturing, BCM includes risk assessment, contingency planning, supply redundancy, and rapid-response protocols to maintain consistent output without compromising quality or compliance.

API supply chains face multiple challenges, including:
These risks highlight the importance of partnering with CDMO pharma companies that can provide secure, resilient supply chain solutions.

CDMOs reinforce API supply chains by building supplier redundancy, maintaining flexible manufacturing at multiple sites, and integrating compliance-focused quality systems. Real-time monitoring enables rapid response to issues, supporting the continuous supply of materials for both clinical and commercial needs. Providers like Piramal Pharma Solutions use their global networks to stabilize the API supply chain.
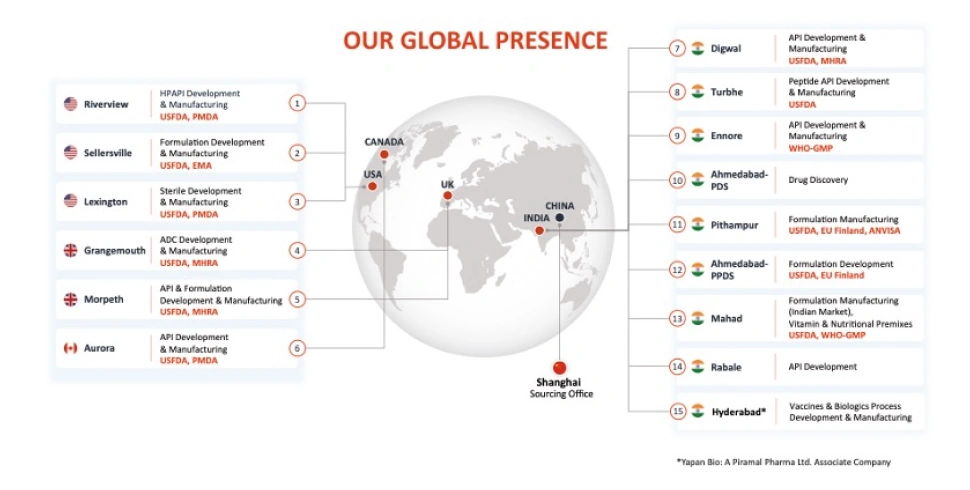
Partnering with a CDMO that prioritizes BCM helps pharmaceutical companies:
A strong BCM program enhances trust, stability, and overall operational resilience


When selecting a pharmaceutical CDMO, companies should consider whether the partner has documented continuity plans, a track record of handling disruptions successfully, redundancy in facilities and raw material sourcing, and systems for real-time monitoring. CDMOs that integrate BCM into API contract manufacturing offer a strong foundation for secure, reliable supply.
Emerging trends include adopting predictive analytics to anticipate supply risks, expanding global redundancy, and integrating digital solutions for real-time monitoring. These strategies will help CDMO pharma companies stay ahead of potential disruptions, ensuring a continuous supply of high potency APIs and other critical drug substances.
It is the proactive planning and processes CDMOs use to maintain consistent API manufacturing services during disruptions.
Supply chains can be affected by limited suppliers, regulatory delays, manufacturing disruptions, and the handling requirements of high potency APIs.
Pharmaceutical CDMOs provide integrated expertise, redundancy, and monitoring systems, helping ensure continuous supply from clinical development through commercialization.
