
search
Dec 03, 2025
In the fast-paced pharmaceutical industry, Contract Development and Manufacturing Organizations (CDMOs) play an essential role in bringing therapies from concept to market. Understanding the types of CDMOs and their capabilities helps developers choose the right partner for each stage of drug development, whether it's early-stage research, clinical production, or full-scale commercialization.
A CDMO is a specialized partner that provides outsourced services in drug development and manufacturing. By combining scientific expertise, manufacturing infrastructure, and regulatory knowledge, a CDMO supports pharmaceutical companies in optimizing processes, scaling production, and ensuring quality. Services can range from small molecule synthesis and biologics production to formulation, analytical testing, and clinical supply management.
CDMOs vary widely based on the scope of services they provide:


These partners specialize in early-stage drug development, including formulation, process design, and preclinical support. They provide technical guidance and help optimize molecules for safety, efficacy, and manufacturability.

Focused primarily on commercial-scale production, these CDMOs offer capabilities for large-scale synthesis, GMP manufacturing, and quality assurance. They ensure consistent, compliant production of APIs and finished drug products.



Full-service or integrated CDMOs combine both development and manufacturing under one roof. They manage projects from discovery through commercial launch, providing seamless transitions between stages, reduced hand-offs, and faster timelines.
The role of CDMOs in pharma is evolving from purely operational partners to strategic collaborators. Today, CDMOs not only handle manufacturing but also contribute scientific expertise, regulatory insight, and process optimization strategies. For complex modalities such as biologics, ADCs, or high-potency APIs, partnering with a full-service CDMO can dramatically improve speed, quality, and efficiency.
At Piramal Pharma Solutions, we offer a full spectrum of CDMO services. This includes both integrated and standalone services across the full drug life cycle, from early-stage discovery and formulation through clinical and commercial manufacturing. Leveraging decades of expertise and an integrated global network, our teams ensure seamless transitions, maintain data continuity, and accelerate timelines. Whether supporting a single stage or managing the full development and manufacturing journey, Piramal delivers the scientific and operational leadership needed to efficiently bring innovative therapies to patients.
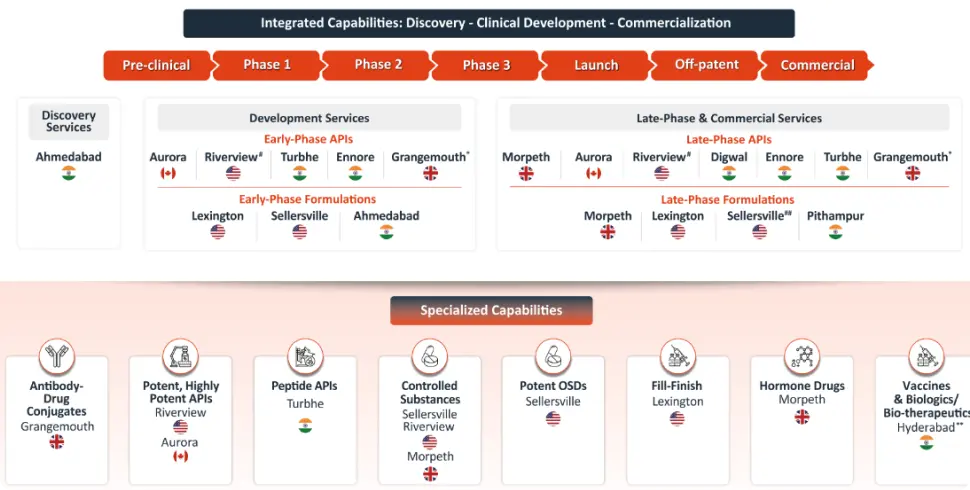
From specialized development CDMOs to full-service end-to-end partners, the CDMO landscape is diverse and strategic. Selecting the right type of CDMO can influence the speed, quality, and success of a drug program. With deep expertise, integrated capabilities, and a global network, Piramal Pharma Solutions exemplifies the modern CDMO, offering flexible, science-driven partnerships that support every stage of pharmaceutical development.
