
search
Jan 13, 2026
Flow chemistry scale-up has become an increasingly important focus in modern drug development programs. Compared to traditional batch processing, flow chemistry offers advantages in speed, safety, process control, and sustainability. Although these benefits are well-documented, many organizations struggle to fully realize them. The challenge is often not adopting flow chemistry in the lab but scaling the process efficiently as molecules move toward clinical and commercial manufacturing.
Successfully translating flow chemistry in drug development from early research into production requires more than technical expertise it depends on integrated capabilities that connect discovery, process development, and manufacturing.


Traditional batch processing remains valuable for slow or well-understood reactions, but it can introduce risks when reactions become unstable, highly exothermic, or difficult to control at scale. As batch volumes increase, heat transfer, pressure management, and safety can become limiting factors.
In contrast, flow chemistry continuously pumps reactants through controlled channels, allowing reactions to proceed under tightly regulated conditions. This makes flow chemistry particularly well-suited for fast reactions, pressure-sensitive chemistries, and photochemical processes. Improved mixing and heat transfer help minimize side reactions, while smaller reactor volumes reduce waste and energy usage, supporting greener pharmaceutical manufacturing.
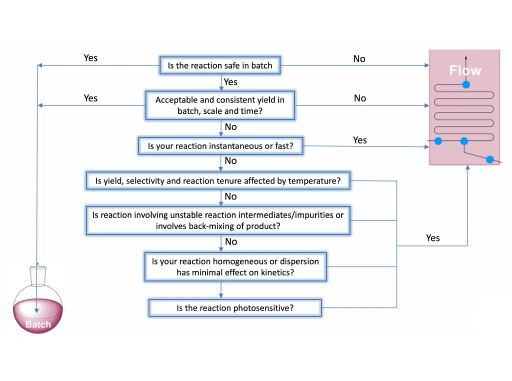
Early evaluation is critical to successful flow chemistry scale-up. During initial process development, benchmarking batch and flow approaches helps teams understand how a synthetic route will perform across the full drug development lifecycle.
Questions around temperature sensitivity, selectivity, pressure requirements, and reaction stability can clarify whether flow chemistry offers meaningful advantages. When the choice is not obvious, working with teams that understand both batch and flow at multiple scales helps reduce uncertainty and avoid rework later in development.

Scaling flow chemistry requires close collaboration among chemists, engineers, and development teams who understand how reactions behave at scale. Integrated drug development capabilities ensure that knowledge generated during discovery is seamlessly transferred to development and manufacturing.
This integration enables faster decision-making, smoother technology transfer, and fewer delays as programs advance toward clinical supply and beyond.
At organizations like Piramal Pharma Solutions, integrated discovery and development teams support efficient flow chemistry scale-up by maintaining continuity across stages. Early flow parameters established during discovery can be adapted and refined during development, reducing risk during scale-up.
In one example, flow chemistry was selected to manage a volatile substrate and elevated pressure requirements – conditions that would have posed safety challenges in batch processing. Ongoing collaboration between discovery and development teams helped address impurities and deliver material on a tight timeline.


For many modern synthetic challenges, flow chemistry offers clear advantages over batch processing. However, its full potential is realized only when supported by integrated capabilities that span discovery through manufacturing. By aligning expertise, infrastructure, and communication, drug developers can accelerate timelines, improve safety, and support more sustainable production of critical therapies.
Flow chemistry is a continuous processing approach in which reactants flow through a reactor under controlled conditions, enabling safer and more efficient synthesis compared to traditional batch methods.
Flow chemistry scale-up requires specialized equipment, a strong understanding of the process, and close coordination between discovery and development teams to maintain reaction control and product quality at higher throughput.
Integrated capabilities enable seamless knowledge transfer from discovery to development, reducing technical risk, accelerating timelines, and improving scalability in pharmaceutical manufacturing.
