
search
Dec 05, 2025
In the dynamic pharmaceutical industry, integrated drug development services are crucial for delivering innovative therapies efficiently and effectively. At Piramal, we leverage our integrated network of 15 global facilities to offer comprehensive solutions from drug discovery and development to commercial manufacturing. By providing integrated solutions that span the entire drug life cycle, we reduce the number of partners our clients need to work with, streamlining the drug development process and enhancing productivity.
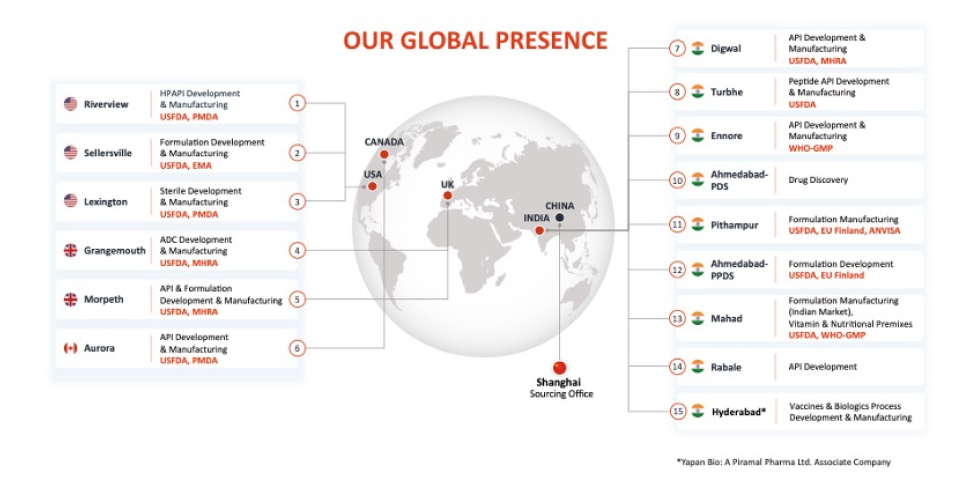


Integrated drug development is a comprehensive strategy that coordinates various elements of the development process under the management of a single partner. This approach minimizes delays, facilitates continuous production, and aligns data and decision-making at every stage.
At Piramal, we provide integrated services that streamline the development process, allowing our clients to concentrate on their core business while we handle the complexities of their programs. By leveraging our extensive network of facilities, we enable smooth transfers between relevant sites, ensuring that projects progress without interruption while optimizing resources and expertise. With extensive experience in all aspects of drug development — from API production to antibody-drug conjugate formulation — we are well-equipped to address all of our clients' challenges in-house.


One of the primary advantages of our integrated approach is enhanced efficiency. By working with a single partner throughout the drug development process, clients benefit from streamlined communication and reduced handoffs. It also minimizes the potential for errors during tech transfers, ensuring that critical information is effectively shared and understood.
Consistency is another significant benefit of choosing an integrated partner. With one partner managing the entire drug life cycle, clients experience a cohesive approach that accelerates project timelines. Each project phase is managed under a unified strategy, allowing quick adaptations and continuous improvements.
Moreover, our integrated solutions enable us to leverage state-of-the-art technologies and methodologies that enhance productivity. The close collaboration among our teams fosters innovation, allowing us to address challenges ly and effectively. This agility is critical in today's competitive landscape, where speed to market can significantly influence a drug's success.
We take a holistic approach to program management to optimize our integration and ensure on-time, complete delivery of overall program goals. Our Project Managers lead cross-functional, site-based teams, driving day-to-day operations to meet objectives. They work closely with our Integrated Program Managers, who oversee the entire program, facilitating seamless transitions and continuous alignment as projects move between sites.
To enhance communication and transparency, we utilize a standardized project management platform consolidating project data from all sites into a user-friendly dashboard. This platform allows customers to access real-time information and customize views to meet their needs. Since 2020, we have completed over 125 integrated drug programs, demonstrating our ability to deliver successful results.


Our commitment to integrated drug development is exemplified by our ADCelerate™ program, which offers a rapid approach to delivering phase-I appropriate ADC drug substance and drug product. By integrating our services — from conjugation development through clinical trial supply and commercial manufacturing done at four distinct sites — we streamline the path from R&D to GMP production.
The ADCelerate™ program showcases the advantages of our integrated approach. By combining our capabilities into a single package of services, we accelerate the timeline from conjugation to scale-up. This enables our clients to initiate clinical trials faster than our competitors, ultimately bringing their innovative therapies to market with speed and precision.
Integrated drug development is a game-changer for the pharmaceutical industry, offering substantial benefits in speed, efficiency, and program management. At Piramal, our commitment to providing integrated solutions helps our clients navigate the complexities of drug development with ease. By facilitating a seamless transition through each phase of the drug life cycle, we empower our partners to focus on delivering innovative therapies to patients in need.
