
search
Jan 28, 2026
Supply chain resilience has become both a competitive advantage and a responsibility in pharmaceutical manufacturing. Over the past two decades, China has grown into a dominant force in the global supply chain, accounting for a significant share of active pharmaceutical ingredient (API) intermediates and raw materials. While this concentration delivered cost and scale benefits, it also introduced risk.
Regulatory changes in China beginning in 2018, followed by the COVID-19 pandemic and rising geopolitical tensions, exposed vulnerabilities tied to geographic dependence. Drug shortages, manufacturing delays, and business continuity risks made one thing clear: supply chain resilience is no longer optional.
As a result, many companies are adopting a China Plus One strategy – a model designed to diversify sourcing while retaining access to China's manufacturing capabilities.


Under a China Plus One model, pharmaceutical companies establish at least one alternate sourcing geography in addition to China. This approach reduces reliance on a single country while maintaining flexibility, cost competitiveness, and supply chain continuity.
The risks of remaining overly dependent are well documented. Supply disruptions can interrupt patient treatment and damage both reputation and revenue. Diversification, by contrast, protects patients and strengthens long-term business performance.
Effective resilience starts with a structured risk assessment. Companies map critical dependencies by product volume, therapeutic importance, and technical complexity, while identifying single-source vulnerabilities and long lead times.
From there, organizations deploy targeted strategies, including:
These actions help ensure quality, transparency, and supply chain continuity while reducing exposure to external shocks.
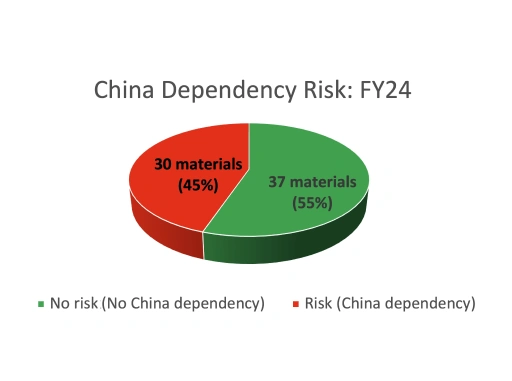
At Piramal Pharma Solutions, supply chain resilience evolved from a focused risk-mitigation initiative into a comprehensive program aligned with sustainability goals. Beginning in 2019, Piramal assessed raw material vulnerabilities, diversified suppliers (particularly in India) and strengthened integrated operations across its global network.

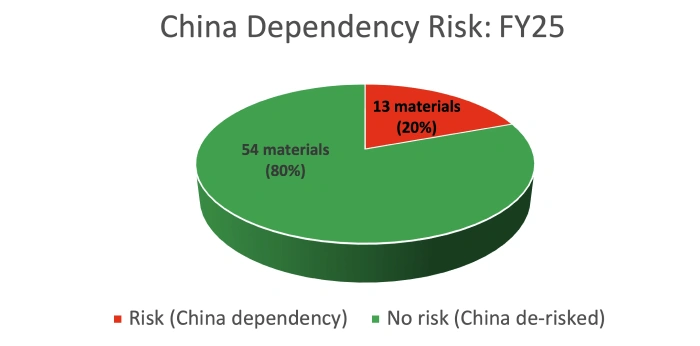

Through systematic risk mapping, Piramal identified dozens of critical materials and prioritized those fully dependent on China. By investing in integrated drug development, backward integration, and technology transfer capabilities, the company rapidly reduced exposure while maintaining an uninterrupted supply.
Within a few years, Piramal significantly reduced its dependence on China, added alternative supply routes for high-risk materials, and maintained cost competitiveness, even during periods of rapid business growth.
Because resilience and sustainability go hand in hand, Piramal has embedded environmental, social, and governance (ESG) considerations into supplier selection and procurement. Using structured assessments, audits, and supplier engagement programs, the company works collaboratively with partners to reduce environmental impact, improve transparency, and strengthen ethical practices across the value chain.
This integrated model supports long-term resilience while advancing responsible sourcing and patient access to medicines.
The China Plus One strategy reflects a broader shift toward resilient, responsible pharmaceutical supply chains. By combining diversification, integrated drug development and manufacturing, and ESG-driven collaboration, companies can protect patients, ensure business continuity, and adapt to an increasingly complex global environment. Piramal's experience shows that resilience is not just about risk avoidance it's about building smarter, more sustainable systems for the future.
China Plus One is a supply chain diversification approach in which companies source materials from China and at least one additional geography to reduce risk.
Supply chain resilience is critical in the pharmaceutical industry because it helps prevent drug shortages, protect patient access to medicines, and reduce business continuity risks.
Integrated drug development and manufacturing enables faster technology transfer, better coordination, and greater control over critical materials across the supply chain.
