
search
Feb 03, 2026
Antibody-drug conjugates (ADCs) represent a cutting-edge approach to targeted therapy, uniting the precision of antibodies with the powerful effects of cytotoxic drugs. As these sophisticated molecules become increasingly important in oncology and other therapeutic fields, their development depends on advanced analytical expertise. ADC pharma programs are highly complex, requiring meticulous characterization, stringent quality control, and comprehensive bioanalytical services to ensure success.
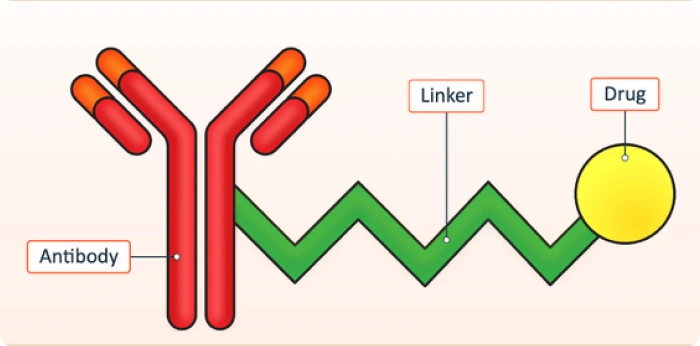

ADCs are complex constructs that consist of an antibody linked to a cytotoxic payload via a chemical linker. This structural complexity creates unique analytical challenges. Variability in drug-to-antibody ratios (DARs), heterogeneity of conjugation sites, and sensitivity to degradation all require a comprehensive analytical assessment. Accurately characterizing these features is critical to ensure safety, efficacy, and regulatory compliance throughout development and manufacturing.
Analytical development underpins every stage of an ADC pharma program. From early stage in silico drug design to preclinical testing and clinical manufacturing, robust analytics guide formulation, process optimization, and quality assurance. Reliable analytical methods allow teams to detect impurities, monitor stability, and predict in vivo performance. Without a strong analytical foundation, scaling ADC GMP manufacturing becomes risky and inefficient.

ADC bioconjugation, or the process of linking the drug payload to the antibody, is particularly challenging. Variability in reaction efficiency and site-specific conjugation can lead to inconsistencies in product quality. Advanced analytical techniques, including mass spectrometry, HPLC, and capillary electrophoresis, are essential to monitor these critical steps. Proper analytical oversight ensures that the final ADC meets specification, maintains potency, and minimizes immunogenicity.
ADC GMP manufacturing relies on precise control and monitoring. Analytical development ensures that every batch aligns with regulatory requirements, reducing the risk of out-of-specification results or production delays. Analytical methods developed during R&D must be scalable, reproducible, and validated to support commercial manufacturing. This approach bridges the gap between laboratory innovation and large-scale ADC production.


Bioanalytical services play a vital role in ADC programs, providing quantitative and qualitative assessments of pharmacokinetics, immunogenicity, and stability. These services guide formulation strategies, optimize dosing regimens, and ensure consistent therapeutic performance. By integrating bioanalytical services expertise early, ADC pharma developers can streamline development timelines and reduce technical risk.
Given the complexity of antibody-drug conjugates, selecting a partner with deep analytical capabilities is essential. Effective partners combine expertise in ADC bioconjugation, ADC GMP manufacturing support, and robust bioanalytical services. By leveraging such a partner, pharmaceutical companies can confidently advance ADC programs from in silico drug design through clinical and commercial stages, ensuring that patients receive safe and effective therapies.
ADCs combine antibodies and cytotoxic drugs, creating heterogeneity in conjugation and variable drug-to-antibody ratios, which require advanced analytical techniques for accurate characterization.
Bioanalytical services provide essential data on pharmacokinetics, immunogenicity, stability, and drug-to-antibody ratios, guiding formulation, dosing, and process development decisions.
Companies should look for a partner with expertise in ADC bioconjugation, ADC GMP manufacturing, bioanalytical services, and scalable methods that support development from R&D through commercial production.
