
search
Dec 23, 2025
Antibody-drug conjugates (ADCs) have emerged as one of the most transformative modalities in oncology, combining the precision of targeted biologics with the potency of cytotoxic drugs. As the demand for safer, more effective cancer therapies accelerates, ADC pharma is experiencing rapid growth – driven by scientific advances, new therapeutic applications, and increasingly sophisticated development strategies.
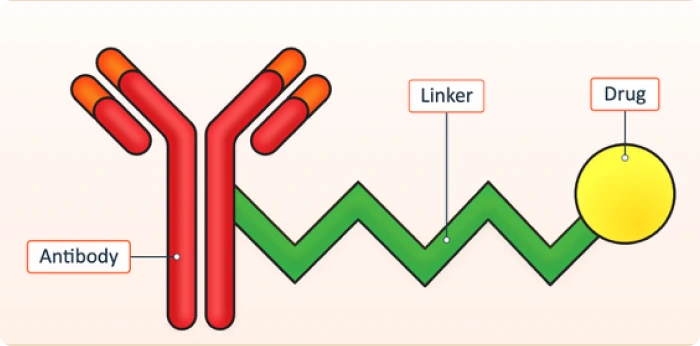

An ADC conjugate consists of three core components:
Together, these elements create a targeted therapy designed to destroy cancer cells while minimizing harm to healthy tissue.
Several factors are driving the rapid expansion of ADC pharma. Improved targeting technologies have increased the safety and specificity of ADCs, while advances in payload potency and linker chemistry are enhancing therapeutic outcomes. Regulatory approvals and clinical successes have validated ADCs as a mainstream oncology tool, driving greater interest in these groundbreaking therapies. Additionally, biopharma innovators are prioritizing precision therapies, accelerating investment in ADC development pipelines.
Recent breakthroughs continue to push the field forward:
These innovations collectively make modern ADCs more stable, more targeted, and more effective than earlier generations.
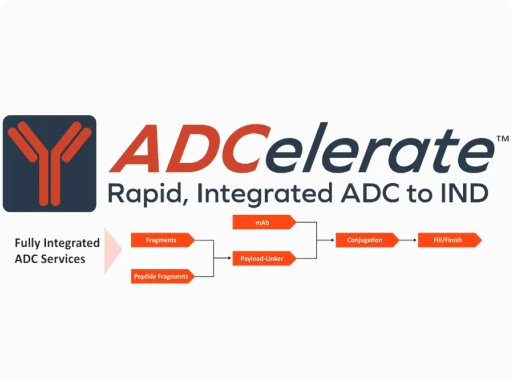

The complexity of ADC development has made integrated solutions essential. Leading ADC services now combine discovery, bioconjugation, analytics, and fill/finish under coordinated project management to accelerate timelines and reduce technical risk.
One example is Piramal Pharma Solutions ADCelerate™ program – a rapid, integrated approach to bioconjugate development. ADCelerate™ streamlines the path from R&D through GMP manufacturing, guaranteeing the delivery of both ADC drug substance and drug product at a fixed price. This model leverages Piramals 20+ years of specialized expertise as pioneers in the ADC space, offering end-to-end support from mAbs and payload–linker development to conjugation, sterile fill/finish, and commercial supply.

ADCs are reshaping cancer care by delivering potent treatments with greater precision. Current applications include:
As targeting strategies evolve, ADCs are expected to expand into even more tumor types and combination therapy regimens.
The future of ADC pharma will center around smarter conjugation technologies, next-gen payloads, and increasingly personalized design, while integrated development platforms, AI-enabled molecular engineering, and improved manufacturability continue to accelerate innovation. As the field advances, ADCs are poised to become a cornerstone of oncology treatment – delivering more targeted, effective, and patient-centric therapeutics. The industry is also seeing application of the technology into therpeutic areas other than oncology.
Antibody-drug conjugates (ADCs) are targeted cancer therapies that link a monoclonal antibody to a potent cytotoxic payload. This allows highly selective delivery of the drug to cancer cells while minimizing systemic exposure.
ADCs are primarily used to treat various forms of cancer, including breast, bladder, lymphoma, and other hard-to-treat tumors. Their precision targeting makes them well-suited for oncology applications.
CDMOs provide specialized capabilities such as conjugation chemistry, payload handling, analytics, and GMP manufacturing. They help innovators streamline and accelerate ADC development with integrated, end-to-end expertise.
