
search
Jan 22, 2026
Antibody-drug conjugates (ADCs) have rapidly evolved into a major oncology therapeutic modality, driven largely by advancements in bioconjugate chemistry and payload-linker design. While early ADC development faced setbacks, recent innovations have unlocked greater selectivity, safety, and clinical success. Today, payload-linker technologies play a defining role in differentiating ADC candidates within increasingly competitive pipelines.
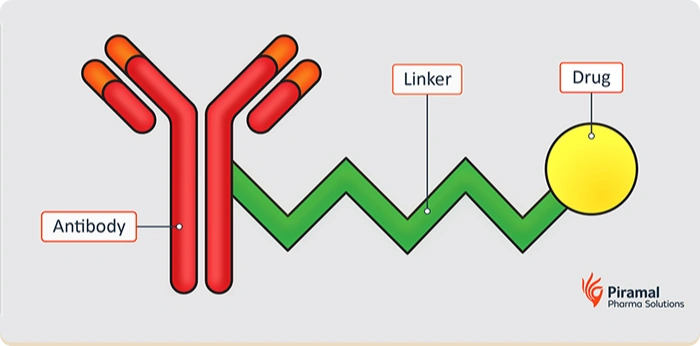

Payload-linkers form the chemical bridge between a targeting antibody and a cytotoxic payload. In ADC drug development, the linker must remain stable in circulation while efficiently releasing the payload once the ADC reaches its target cell. Achieving this balance is critical to optimizing efficacy while minimizing off-target toxicity.
Linkers are generally categorized as cleavable or non-cleavable. Cleavable linkers release payloads in response to specific biological triggers, such as enzymes present in the tumor microenvironment. Non-cleavable linkers rely on intracellular antibody degradation to release the payload, offering enhanced stability and reduced risk of premature release. Each approach carries tradeoffs that directly impact safety, potency, and tumor selectivity.
As competition intensifies across shared ADC targets, developers must design highly differentiated payload-linker systems to succeed. According to industry tracking, the number of ADCs in clinical development increased by more than 30% between 2018 and 2023, underscoring the need for innovation and speed within each drug development program.
The diversity of available payloads and linker chemistries enables a wide range of rational design strategies but also increases complexity. Selecting the optimal combination requires deep technical expertise and experience across multiple ADC constructs.



Leading ADC CDMO partners support innovation by providing access to custom, non-commercial payload-linkers, tailored to specific therapeutic goals. Rather than relying on off-the-shelf components, custom design allows developers to fine-tune stability, release mechanisms, and payload compatibility.
Piramal Pharma Solutions supports ADC innovation through custom payload-linker development at its Riverview, Michigan API/HPAPI site. The facility has extensive experience handling both standard and high-potency payload-linkers, including chemistries designed for enzyme-cleavable, pH-sensitive, and multi-payload attachment strategies. This work supports a broad range of cytotoxic payloads and enables scalable production under controlled conditions.
Payload-linker development is most effective when integrated into a broader integrated drug discovery and manufacturing strategy. Coordinated workflows across discovery, development, conjugation, and fill/finish can significantly shorten timelines and reduce risk.
Piramal';s payload-linker capabilities are part of its integrated ADC service platform, ADCelerate™, which connects upstream intermediate synthesis, payload-linker assembly, conjugation, and sterile manufacturing across multiple global sites. This model supports parallel development activities, accelerates delivery of clinical and GMP materials, and enables efficient scale-up from early research to commercialization.
Payload-linker technologies are a critical differentiator in modern ADC development, shaping safety, selectivity, and therapeutic performance. As ADC pipelines continue to expand, partnering with CDMO biologics services providers that offer deep payload-linker expertise and integrated capabilities can help developers move faster, reduce complexity, and bring differentiated cancer therapies to patients.
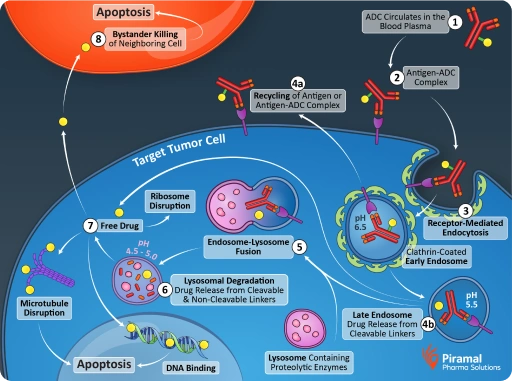
Payload-linkers control when and how cytotoxic drugs are released, directly impacting ADC safety and efficacy.
Custom payload-linker designs allow ADC developers to differentiate candidates and optimize performance for specific targets.
ADC CDMOs provide specialized chemistry expertise, HPAPI handling, and integrated services across drug development and manufacturing.
