
search


The pharmaceutical industry faces increasing pressure to deliver innovative therapies rapidly while embracing sustainable, environmentally responsible practices that ensure efficiency and safety throughout the drug development process. This white paper demonstrates how integrating green chemistry principles at the discovery stage delivers both scientific and strategic advantages for organizations aiming to improve efficiency and sustainability.
-1-1.png)
Seeking to mitigate increasing supply chain risks, pharmaceutical companies have identified “China Plus One” as a strategic imperative. Under this risk-management model, companies diversify their supply chains to include at least one other country in addition to China, allowing them to continue benefitting from China';s manufacturing footprint while avoiding the risks associated with relying solely on one country.
-3.png)
Cryogenic reactions are essential for synthesizing certain active pharmaceutical ingredients (APIs), enabling chemists to reduce impurities, ensure safety, improve selectivity, and facilitate reactions that are impossible at higher temperatures. The advantages of cryogenic conditions make them critical to the development and production of life-changing medicines.
-2-1.png)
An increased focus on targeted therapeutics has driven rapid growth in the Highly Potent Active Pharmaceutical Ingredients (HPAPIs) segment of the pharmaceutical industry. The surge in antibody-drug conjugates (ADCs) boosts that forecast even further since their core active component, the payload, is typically classified as an HPAPI.
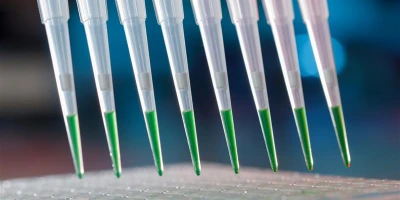
High-throughput experimentation (HTE) empowers drug developers to quickly build robust platforms for scale-up. Facing constraints on their time, resources, and materials, companies leverage this approach to rapidly execute many miniaturized reactions in parallel to survey a wide range of possible reaction conditions.
